Produce work to explain the Ideal Gas Law, its equation and how to use it. Your response can be a presentation or a piece of text. You can upload Word Documents. Power Point files or videos. The criteria are to be independent, original, informative and entertaining. Include 2 questions that everyone else can respond to. REMEMBER TO CITE YOUR SOURCES.
- Comment
- Reblog
-
Subscribe
Subscribed
Already have a WordPress.com account? Log in now.

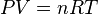
Ideal Gas Law
Gases never behave in an ideal way to then be able to work with them in formulae. When it comes to Kinetic Theory (explaining the properties of states of matter), some are assumptions are made about gases to make the use of formulae easier. Gases do not behave in the way I am to describe but some of them work in an approximate way as if they were at ordinary temperature and pressure. Some of the assumptions made are:
• The random motion of the gas molecules obey Newton’s Law of Motion; they also travel in straight lines
• All intermolecular collisions are perfectly elastic
• No forces act of the molecules except in elastic collisions
• The volume of the gas molecules is negligible in comparison to the volume of space that these molecules occupy
• There are no intermolecular forces between each molecule of gas
• The kinetic energy of the molecules is proportional to the temperature at which they are kept
The equation for the ideal gas law reads:
PV=nRT
where:
• P = Pressure (in Pascals [Pa])
• V = Volume (in metres cubed [m³])
• n = Number of moles
• R = The Gas constant (8.31441 J K-1 mol-1)
• T = Temperature (in Kelvin [K (i.e. °C+273)])
The formula in use:
How much space would 3 moles of a gas occupy at room temperature and pressure, knowing that room temperature is 21°C and pressure is 1 atm?
1 atm = 101.325 kPa
1) State values and convert units
P=101.325kPa x1000 => 101325Pa. V=x (the unknown). n=3. R=8.31441JK-1mol-1
T=21°C + 273 => 294K
2) Input into formula
PV=nRT
101325xX = 3×8.31441×294
3) Rearrange and calculate
X = 3×8.31441×294
101325
X = 0.0724m³ (to 3 sig.fig.)
Or multiply by 1000 so it is 72.4dm³
Practice Q’s
How much space would 2 moles of a gas occupy at room pressure and 273K, knowing that room pressure is 1 atm?
1 atm = 101.325 kPa
How many moles are there in 84.3dm³ of gas at room pressure and at 45°C, to the nearest mole? Knowing that room pressure is 1 atm and 1 atm = 101.325 kPa
Sources
http://abyss.uoregon.edu/~js/glossary/ideal_gas_law.html
http://chemguide.co.uk/physical/kt/idealgases.html
LikeLike
1)0.045dm^3
2)3230mol
LikeLike
Yes, but I recommend using the same rounding for both your answers. Either 2 d.p. or 3-4 sig fig 🙂
LikeLike
In fact, after reviewing, you fell into a trap I feel into myself when responding to your answer. Check question 2 again and ensure you have used the values in the correct units to the equation PV=nRT!!
LikeLike
1)0.0448dm^3
2)3230.6mol
LikeLike
Yes, but the same as I said to Jonathan, probably 3 sig fig for your answers 🙂
LikeLike
Just as Jonathan did, you have fallen into a trap I fell into myself when responding to your answer. Check question 2 again and ensure you have used the values in the correct units to the equation PV=nRT!!
LikeLike
The Ideal Gas Law
The Ideal gas law is the law which shows the correspondence between the pressure, temperature, volume and molecules/moles of a gas based on the assumptions that we make that all gases have.
For gases we assume that they are made up of molecules which are in constant random motion in straight lines. Pressure is due to collisions between the molecules and the walls of the container. All collisions, both between the molecules themselves, and between the molecules and the walls of the container, are perfectly elastic so there is no loss of kinetic energy during the collision. Also, that the temperature of the gas is proportional to the average kinetic energy of the molecules.
PV=nRT=NkT
P=Pressure in Pa
V=Volume in
n=number of moles
N=Number of molecules
N-A=Avogadro’s number 6.023 x 10-23
K=1.38066 x 10-23
J/K=8.617385 x 10- -5
eV/K
k=R/N-A
R=Universal gas constant 8.1345 J/mol K
Temperature is proportional to pressure and therefore kinetic energy so this proves the idea of kinetic temperature.
http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/idegas.html
http://www.chemguide.co.uk/physical/kt/idealgases.html
LikeLike
pV=nRT – The ideal gas equation.
The ideal gas law comes from the simple gas laws which are Boyle’s Law, Charles’ Law and Avogadro’s Law. Boyle’s law describes the inversely proportional relationship between pressure and volume. This can be shown as P ∝ 1/V. Charles’ Law describes the directly proportional relationship between volume and temperature of a fixed amount of gas when pressure is constant. This can be shown as V ∝ T. Avogadro’s law is that the volume of gas is directly proportional to the amount (in moles of gas) at a constant temperature and pressure.
Amontons law is also useful which is that pressure is directly proportional to temperature at a constant number of moles of gas and an unchanged volume.
What it means:
Pressure * volume = number of moles * gas constant * temperature
Measurements
(Pascals, atmospheres, Hg (Torr) * (dm-3 , m3, l)= moles *8.3144598 J mol-1 K-1* K
The Gas constant can also be:
0.082057 L atm mol-1 K-1
62.364 L Torr mol-1 K-1
8.3145 m3 Pa mol-1 K-1
These values are all just different measurements of pressure.
Standard Temperature and Pressure: The universal value of this is generally given as 1 atmosphere and 0 0 C. This value has to be changed into Kelvin.
The ideal gas Equation is used to solve a problem when you know the amount of gas given and that the mass of the gas is constant. You could then be asked to solve for the unknown variable. You must make sure to match your units for pressure and the gas constant (pascals or atmospheres etc.).
Once you are given the values, you then must rearrange the formula in order to look for the designated variable.
e.g. Looking for temperature: pV=nRT
pV/nR=T
Once done, put in the values in order to determine your value. Remember to make sure your pressure and constant match and that you change your units to the appropriate value, for example temperature to K for your working out.
Question 1: What is the volume (in litres) of Oxygen when the pressure is 1.2atm, at a temperature of 250C when you have 8g of Oxygen?
Question 2: What is the temperature of the 16m3 cylinder when there is 110g of CO2 at a pressure of 11 Pa?
http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/idegas.html
http://www.chemguide.co.uk/physical/kt/idealgases.html
http://chemwiki.ucdavis.edu/Physical_Chemistry/Physical_Properties_of_Matter/Phases_of_Matter/Gases/Gas_Laws/The_Ideal_Gas_Law
LikeLike
1) 10.19l (Presuming the temperature was 25 degrees C)
2) 8.47K
LikeLike
https://kayleighjeffrey.files.wordpress.com/2015/09/ideal-gas-law1.odt
LikeLike
Ideal Gas Law
The Ideal Gas Law was devised in 1834 and was first stated by Emile Clapeyron and is a way to understand the relationship between gas molecules and their environment without the inclusion of other molecular forces. The equation: PV = nRT, takes into account elements such as number of moles, volume, pressure etc.
P = Pressure (Pa)
V = Volume (m3)
n = Number of Moles
R = Gas Constant (8.31441 J K-1 mol-1
T = Temperature (K)
Question 1:
9.0 g of chlorine is at 289 mm Hg and at a temperature of 27º C. What is the volume?
Question 2:
What is a gas’s temperature in Celsius when it has a volume of 36 L, 194 mol, 145 atm?
References
http://www.chemguide.co.uk/physical/kt/idealgases.html
http://chemwiki.ucdavis.edu/Physical_Chemistry/Physical_Properties_of_Matter/Phases_of_Matter/Gases/Gas_Laws/The_Ideal_Gas_Law
LikeLike
Question 2:
What is a gas’s temperature in Celsius when it has a volume of 36 L, 194 mol, 145 atm?
54.7 C
LikeLike
a= 0.19 to 2 d.p. I did PV=nRT moles of chlorine me n+m/M = 9.0g/35.5 = 0.25 mol. o.25*8.31441*27/289mm HG =0.1969…
LikeLike
The Ideal Gas Law
An ideal gas can be described as a gas that contains perfectly elastic collisions between the atoms or molecules. [1] Obviously, this is a hypothetical situation, as there is no such thing as an ideal gas. However, it is a good approximation to the behavior of most gases under most conditions, despite the limitations.
The ideal gas equation is:
PV=nRT
Where:
n= number of moles
R= universal gas constant (8.13145 J/mol K)
P= pressure (in Pa)
V= Volume (in m3)
T= Temperature (in K)
Many kinetic theory assumptions are made about ideal gases. The assumptions are:
• Gases are made up of molecules that are in constant random, straight-line motion.
• All collisions are perfectly elastic (no loss of KE)
• There are no intermolecular forces between the gas molecules.
• The temperature of the gas is proportional to the average kinetic energy of the molecules.
• The molecules behave as rigid spheres. [2]
Example Question:
If we had 1.0mol of gas at 1.0 atm of pressure at 0 degrees Celsius, what would the volume be?
Step 1- rearrange the formula
V= nRT/P
Step 2- convert temperature to Kelvin- 0 degree Celsius= 273K
Step 3- input the numbers
V= 1 x 0.0821 / 1
= 22.41m3
* 0 degrees Celsius and 1atm pressure are referred to as the standard temperature and pressure (STP)
Practice Questiin:
If you had 750 ml of gas, pressure of 2.8 atmospheres and a temperature of 53.6 °C, how many moles of gas do you have?
References:
[1] http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/idegas.html
[2] http://www.chemguide.co.uk/physical/kt/idealgases.html
LikeLike
If you had 750 ml of gas, pressure of 2.8 atmospheres and a temperature of 53.6 °C, how many moles of gas do you have?
n = pv/t
p=2.8
v=750
t=53.6
0.8 moles
LikeLike
The ideal gas law
The ideal gas law is the equation of state of a hypothetical ideal gas. It’s a good approximation to the behaviour of many gases under many conditions. However it has its limitations. The theory was first put forward by Émile Clapeyron in 1834 by using Boyles law, Charles law and Avogadro’s law.
An ideal gas is a model of real gases which do follow ideal gas behavior if their density is low enough that the molecules don’t interact much. When they do collide they undergo an elastic collision (where the kinetic energy is the same before and after the collision).
Ideal Gas Law: pV = nRT.
P= Pressure (Pa)
V= Volume (m3)
n= Number of moles
R= The gas constant (8.13145 J/mol K)
T= Temperature (K)
Example Question
Calculate the volume occupied by 1 mole of a gas at 25 oC and 100 kPa.
Convert into the base units needed.
25 + 273 = 298K
100 x 1000 = 100,000Pa
Re-arrange the formula
V = nxRxT/P
Substitute numbers into the equation
V = 1×8.13145×298/100,000
=0.024m3
Practice Question
Calculate the pressure of a gas given that 0.2 moles of the gas occupy 10 dm3 at 20 oC.
http://www.chemguide.co.uk/physical/kt/idealgases.html
http://www.a-levelchemistry.co.uk/…/1.2%20Exercise%203%20-%20ideal%20...
LikeLike
1) 47.65 Pa
LikeLike
48.7Pa
LikeLike
P= ntr/v 20+273=293K ( temp) 0.2 mol (moles) 10 dm3 (vloume ) ( do you have to convert thisto dm3 all the time?) 0.2*8.13145*293/10= 47.67 Pa
LikeLike
From Emily Kirkham
https://dykehouseyear12chem.wordpress.com/emily-kirkham-gas-law-ppt/
LikeLike
PV=nRT
This is the ideal gas law, however it could be argued as incorrect as you have to make 2 assumptions :
the particles have no forces acting on them.
these particles do not take up any space.
This could cause controversy and would alter the results if these were took into consideration.
Variables:
P = Pressure
V=Volume
n=Number of moles
T=Temperature
R=Gas constant
You can solve any gas equation using this equation which why it is named as the ideal gas law.
It is a mix of Boyles’, Charles’, and Avogadro’s laws and has been combined to make the perfect equation and is used worldwide by all scientists.
1 mol of any gas at 0 Celsius and 1 atm has a volume of 22.4 L. The conditions 273 K and 1 atm are the standard temperature and pressure for a gas.
references:
http://chemwiki.ucdavis.edu/Physical_Chemistry/Physical_Properties_of_Matter/Phases_of_Matter/Gases/Gas_Laws/The_Ideal_Gas_Law
http://www.sparknotes.com/chemistry/gases/ideal/section2.rhtml
LikeLike
PV=nRT
This is the ideal gas law, however it could be argued as incorrect as you have to make 2 assumptions :
the particles have no forces acting on them.
these particles do not take up any space.
This could cause controversy and would alter the results if these were took into consideration.
Variables:
P = Pressure
V=Volume
n=Number of moles
T=Temperature
R=Gas constant
You can solve any gas equation using this equation which why it is named as the ideal gas law.
It is a mix of Boyles’, Charles’, and Avogadro’s laws and has been combined to make the perfect equation and is used worldwide by all scientists.
1 mol of any gas at 0 Celsius and 1 atm has a volume of 22.4 L. The conditions 273 K and 1 atm are the standard temperature and pressure for a gas.
Practice question:
Calculate the pressure of a gas given that 0.5 moles of the gas occupy 20 dm3 at 5 oC.
LikeLike
56Pa
LikeLike
56.51 Pa
LikeLike
The Ideal Gas Law is very simply expressed:
PV=nRT
From which simpler gas laws such as Boyle’s, Charles’s, Avogadro’s and Amonton’s law be derived.
When dealing with gas, a famous equation was used to relate all of the factors needed in order to solve a gas problem. This equation is known as the Ideal Gas Equation. As we have always known, anything ideal does not exist. In this issue, two well-known assumptions should have been made beforehand:
The particles have no forces acting among them, and
These particles do not take up any space, meaning their atomic volume is completely ignored.
The four gas variables are: pressure (P), volume (V), number of mole of gas (n), and temperature (T). Lastly, the constant in the equation shown below is R, known as the the gas constant
A few things should always be kept in mind when working with this equation, as you may find it extremely helpful when checking your answer after working out a gas problem.
Pressure is directly proportional to number of molecule and temperature. (Since P is on the opposite side of the equation to n and T)
Pressure, however, is indirectly proportional to volume. (Since P is on the same side of the equation with V)
Boyle’s Law-
Boyle’s Law describes the inverse proportional relationship between pressure and volume at a constant temperature and a fixed amount of gas. This law came from a manipulation of the Ideal Gas Law.
P∝1V
P1V1=P2V2
Charles’s Law-
Charles’s Law describes the directly proportional relationship between the volume and temperature (in Kelvin) of a fixed amount of gas, when the pressure is held constant.
V∝T
V1T1=V2T2
Avogadro’s Law-
Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure.
V∝n
V1n1=V2n2
Amontons’s Law-
Given a constant number of mole of a gas and an unchanged volume, pressure is directly proportional to temperature.
P∝T
P1T1=P2T2
Standard Temperature and Pressure (STP)-
The universal value of STP is 1 atm (pressure) and 0o C. Note that this form specifically stated 0o C degree, not 273 Kelvin, even thought you will have to convert into Kelvin when plugging this value into the Ideal Gas equation or any of the simple gas equations.
In STP, 1 mole of gas will take up 22.4 L of the volume of the container.
P = Pressure (in Pascals [Pa])
V = Volume (in metres cubed [m³])
n = Number of moles
R = The Gas constant (8.31441 J K-1 mol-1)
T = Temperature (in Kelvin [K (i.e. °C+273)])
source: http://chemwiki.ucdavis.edu/Physical_Chemistry/Physical_Properties_of_Matter/Phases_of_Matter/Gases/Gas_Laws/The_Ideal_Gas_Law
LikeLike